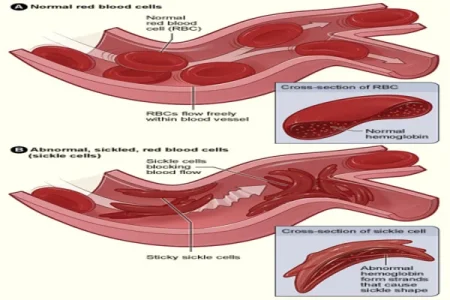
Image Credit: NHLBI
In a historic move, the Food and Drug Administration (FDA) has granted approval for Casgevy, a powerful treatment for sickle cell disease, affecting over 100,000 Americans, primarily from the Black community.
Developed by Vertex Pharmaceuticals and CRISPR Therapeutics, this therapy marks the first-ever FDA approval of a medicine using the revolutionary gene-editing tool CRISPR, recognized with the Nobel Prize in Chemistry in 2020.
Dr. Alexis Thompson, Chief of Hematology at Children’s Hospital of Philadelphia, describes this moment as pivotal in the medical field. Casgevy, designed for individuals aged 12 and older, emerges as a game-changer by removing the need for a donor. Using CRISPR, the treatment edits the DNA in a patient's stem cells, eliminating the gene responsible for sickle cell disease. Notably, patients become their own donors, representing a significant shift in treatment dynamics.
While hailed as revolutionary, the treatment comes with a substantial price tag of $2.2 million per patient. Dr. Rabi Hanna from the Cleveland Clinic emphasizes the importance of ensuring accessibility to make this groundbreaking therapy available to a broader population, potentially serving as an equalizer for those with sickle cell disease.
The FDA's decision includes the approval of another treatment, Lyfgenia, a gene therapy from Bluebird Bio, offering two potential breakthroughs for this inherited blood disorder. Both treatments focus on genetically modifying a patient's own stem cells, eliminating the need for external donors, a process fraught with challenges like rejection and finding suitable matches.
Casgevy's approval is met with widespread optimism from healthcare professionals, with Dr. Asmaa Ferdjallah, a pediatric hematologist, calling it a "game-changer" that reimagines sickle cell disease as a curable condition. However, experts highlight concerns about the treatment's hefty price tag, potentially limiting accessibility for many families. Dr. Hanna stresses the importance of making the therapy accessible, recognizing its potential to empower individuals with sickle cell disease to pursue various career options.
The treatment process involves multiple phases spanning several months. Patients undergo a series of blood transfusions, stem cell extraction, and CRISPR editing in a laboratory. The edited stem cells are then reinfused after chemotherapy to ensure the removal of flawed cells. Dr. Hanna emphasizes the necessity of managing patient expectations during this complex journey.
The clinical trial, involving 46 participants globally, reports success in 29 cases, demonstrating the effectiveness of Casgevy. LaRae Morning, one of the trial patients, shares her transformative experience, emphasizing how the therapy has allowed her to lead a normal life, participating in activities that were once restricted by sickle cell disease.
Despite the remarkable progress, questions about potential long-term effects persist. The FDA advisory committee discusses concerns about "off-target" effects, where the gene-editing tool inadvertently affects other stretches of DNA. While the trial's current span is two years, Dr. Monica Bhatia emphasizes the need for extended follow-up to monitor for any unforeseen consequences.
The approval of Casgevy represents a significant leap forward in the treatment landscape for sickle cell disease, offering hope and potential relief to those affected. The medical community anticipates continued advancements and vigilance in ensuring the therapy's long-term safety and accessibility.